Bioassay


Batch release testing
Since more than 15 years we offer efficacy testing of drugs under GMP quality standards at our site. The Erythropoietin (EPO) Bioassay is regularly performed following the specifications stated in the European Pharmacopeia (Ph. Eur.).
For our customers we perform regular batch release and stability testing as well as (transfer) validation of customer specific methods to support you as a back-up laboratory or to evolve a specific test procedure for your product.
GMP Testing
GMP Quality Control
Method Development & Validation






Dissolution
Extractables & Leachables






Particle Determination
Elemental Impurities Testing



Regulatory Services
Amino Acid Analysis




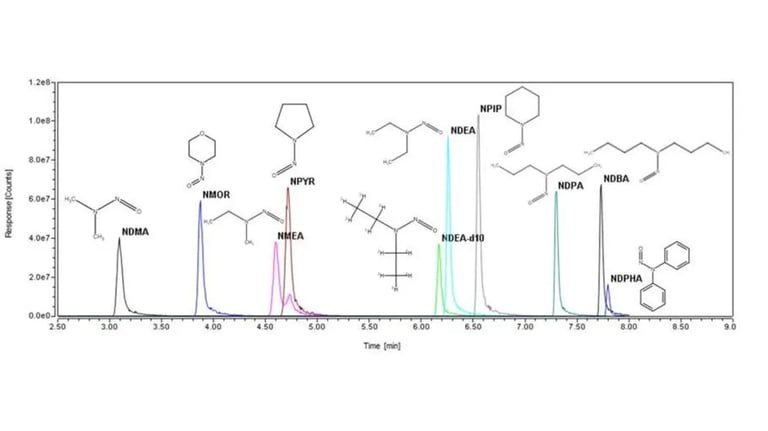
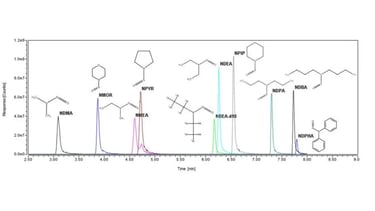
N-nitrosamines
qPCR




Protein Characterization


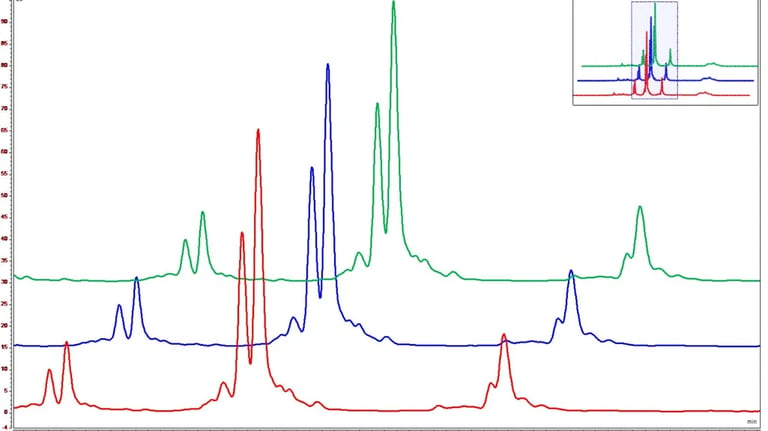
Bioassay
Stability Studies
Packaging Material Testing
Microbiology
Instrumentation
cIEF
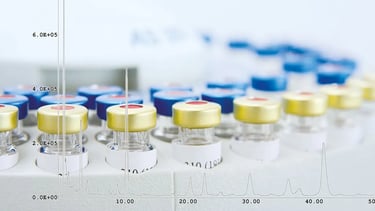
Limit test for DEG and EG by GC
Our Services
The experienced and highly committed GBA Pharma Labs team offers a variety of different services in the following Areas.


