Pharmacopoeial Testing


Experience
Pharmacopoeial Testing – Precise, Reliable, to the Point
Pharmacopoeial methods are part of our daily routine – and it shows. Whether Ph. Eur. USP/NF, or JP: we test your products in full compliance with regulatory requirements. Our Focus? Accuracy, efficiency, and clear communication.
What do we test? Everything that matters: identity, purity, content, uniformity, dissolution, and more.
Curious? Explore our range of pharmacopoeial testing services.
Packing Materials Testing
Glass containers
Ph. Eur. 3.2.1
USP/NF 〈660〉
Plastic containers
Ph. Eur. 3.2.3
Ph. Eur. 3.2.4
Ph. Eur. 3.2.5
Ph. Eur. 3.2.6
USP/NF 〈661〉
USP/NF 〈671〉
Rubber Seals / Silicon Elastomers
Ph. Eur. 3.2.9
USP/NF 〈381〉
Ph. Eur. 3.1.9
Bioanalytical Services and Microbiology
Ph. Eur. 2.2.31 Electrophoresis
Ph. Eur. 2.2.44 Total organic carbon in water for pharmaceutical use
Ph. Eur. 2.2.47 Capillary electrophoresis
Ph. Eur. 2.5.33 Total protein
Ph. Eur. 2.6.7 Mycoplasmas
Ph. Eur. 2.6.12 Microbiological examination of non-sterile products: microbial enumeration tests
Ph. Eur. 2.6.13 Microbiological examination of non-sterile products: test for specified micro-organisms
Ph. Eur. 2.6.14 Bacterial endotoxins
Ph. Eur. 2.6.30 Monocyte-activation test
Ph. Eur. 2.6.31 Microbiological examination of herbal medicinal products for oral use and extracts used in their preparation
Ph. Eur. 2.6.34 Host-cell protein assay
Ph. Eur. 2.6.35 Quantification and characterisation of residual host-cell DNA
Ph. Eur. 2.7.2 Microbiological assay of antibiotics
Ph. Eur. 2.7.5 Assay of heparin
Ph. Eur. 5.1.3 Efficacy of antimicrobial preservation
Ph. Eur. 5.1.4 Microbiological quality of non-sterile pharmaceutical preparations and substances for pharmaceutical use
Ph. Eur. 5.1.8 Microbiological quality of herbal medicinal products for oral use and extracts used in their preparation
USP/NF 〈51〉 Antimicrobial Effectiveness Testing
USP/NF 〈60〉 Microbiological Examination of Nonsterile Products Tests for Burkholderia Cepacia Complex
USP/NF 〈61〉 Microbiological Examination of Nonsterile Products: Microbial Enumeration Tests
USP/NF 〈62〉 Microbiological Examination of Nonsterile Products: Tests for Specified Microorganisms
USP/NF 〈81〉 Antibiotics—Microbial Assays
USP/NF 〈85〉 Bacterial Endotoxins Test
USP/NF 〈507〉 Protein Determination Procedures
USP/NF 〈509〉 Residual DNA Testing
USP/NF 〈643〉 Total Organic Carbon
USP/NF 〈1103〉 Immunological Test Methods—Enzyme-Linked Immunosorbent Assay (Elisa)
USP/NF 〈1104〉 Immunological Test Methods—Immunoblot Analysis
USP/NF 〈1111〉 Microbiological Examination of Nonsterile Products: Acceptance Criteria for Pharmaceutical Preparations and Substances for Pharmaceutical Use
USP/NF 〈1126〉 Nucleic Acid-Based Techniques—Extraction, Detection, and Sequencing
USP/NF 〈1127〉 Nucleic Acid-Based Techniques—Amplification
USP/NF 〈1130〉 Nucleic Acid-Based Techniques—Approaches for Detecting Trace Nucleic Acids (Residual DNA Testing)
Physicochemical analyses
Assay
Ph. Eur. 2.5.1. Acid value
Ph. Eur. 2.5.3. Hydroxyl value
Ph. Eur. 2.5.6. Saponification value
Ph. Eur. 2.5.11. Complexometric titrations
Ph. Eur. 2.5.12. Water: semi-micro determination
Ph. Eur. 2.5.32. Water: micro determination
Ph. Eur. 2.5.29. Sulfur dioxide
Ph. Eur. 2.5.30. Oxidising substances
Ph. Eur. 2.5.33. Total Protein e. g. Bradford,
Lowry
USP/NF 〈921〉 Water Determination
USP/NF 〈401〉 Fats and Fixed Oils
USP/NF 〈525〉 Sulfur Dioxide
USP/NF 〈507〉 Protein Determination Procedures e. g. Bradford, Lowry
Limit tests
Ph. Eur. 2.4.3. Calcium
Ph. Eur. 2.4.4. Chlorides
Ph. Eur. 2.4.6. Magnesium
Ph. Eur. 2.4.8. Heavy metals
Ph. Eur. 2.4.9. Iron
Ph. Eur. 2.4.13. Sulfates
Ph. Eur. 2.4.14. Sulfated ash
Ph. Eur. 2.4.16. Total ash
Acidity or alkalinity
reducing substances
USP/NF 〈281〉 Residue on Ignition
USP/NF 〈221〉 Chloride and Sulfate
USP/NF 〈231〉 Heavy Metals
Titrations
Ph. Eur. 2.2.20. Potentiometric titration
USP/NF 〈541〉 Titrimetry
Ph. Eur. 2.5.11. Complexometric titrations
Ph. Eur. 2.5.12. Water: semi-micro determination
Ph. Eur. 2.5.32. Water: micro determination
USP/NF 〈921〉 Water Determination
Viscosity
Ph. Eur. 2.2.9. Capillary viscometer method
Ph. Eur. 2.2.10. Viscosity - Rotating viscometer method
USP/NF 〈1911〉 Rheometry
others Ph. Eur. 2.2.32. Loss on drying
USP/NF 〈731〉 Loss on Drying
Ph. Eur. 2.3.1. Identification reactions of ions and functional groups
USP/NF 〈191〉 Identification Tests—General
Ph. Eur. 2.9.1. Disintegration of tablets and capsules
Ph. Eur. 2.9.2. Disintegration test for solid rectal and vaginal dosage forms
USP/NF 〈701〉 Disintegration
Ph. Eur. 2.2.5. Relative density
USP/NF 〈841〉 Specific Gravity
Ph. Eur. 2.2.6. Refractive index
USP/NF 〈831〉 Refractive Index
Ph. Eur. 2.9.8. Resistance to crushing of tablets
USP/NF 〈1217〉 Tablet Breaking Force
Ph. Eur. 2.2.7. Optical rotation
USP/NF 〈781〉 Optical Rotation
Ph. Eur. 2.8.4. Swelling index
Ph. Eur. 2.2.35. Osmolality
USP/NF 〈785〉 Osmolality and Osmolarity
Ph. Eur. 2.9.39. Water-solid Interactions: determination of sorption-desorption isotherms and of water activity
USP/NF 〈922〉 Water Activity
Freisetzung und Gleichförmigkeit
Dissolution and uniformity
Dissolution
Ph. Eur. 2.9.3. Dissolution test for solid dosage forms
Ph. Eur. 2.9.4. Dissolution test for patches
Ph. Eur. 2.9.25. Dissolution test for medicated chewing gums
Ph. Eur. 2.9.42. Dissolution test for lipophilic solid dosage forms
Ph. Eur. 2.9.43. Apparent dissolution
USP/NF 〈711〉 Dissolution
Uniformity
Ph. Eur. 2.9.5. Uniformity of mass of single-dose preparations
Ph. Eur. 2.9.6. Uniformity of content of single-dose preparations
Ph. Eur. 2.9.40. Uniformity of dosage units
USP/NF 〈905〉 Uniformity of Dosage Units
ICP-MS / HPLC / GC
Assay, identity and limit tests
Examples
General
Ph. Eur. 2.2.27. Thin-layer chromatography
Ph. Eur. 2.2.28. Gas chromatography
Ph. Eur. 2.2.29. Liquid chromatography
Ph. Eur. 2.2.30. Size-exclusion chromatography
Ph. Eur. 2.2.46. Chromatographic separation techniques
Ph. Eur. 2.2.47. Capillary electrophoresis
Composition of fatty acids
Ph. Eur. 2.4.22. Composition of fatty acids by gas chromatography
USP/NF 〈401〉 Fats and Fixed Oils
residual solvents
Ph. Eur. 2.4.24. Identification and control of residual solvents
USP/NF 〈467〉 Residual Solvents
USP/NF 〈1467〉 Residual Solvents—Verification Of Compendial Procedures And Validation Of Alternative Procedures
elemental impurities / Screening
Ph. Eur. 2.4.20. Determination of elemental impurities
USP/NF 〈232〉 Elemental Impurities—Limits
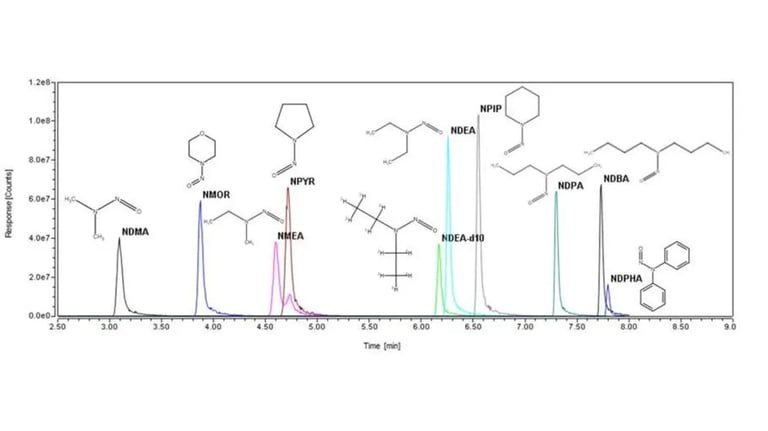
N-Nitrosamines
untargeted screening
Amino Acids
Ph. Eur. 2.2.56. Amino acid analysis
ethylene oxide and dioxan
Ph. Eur. 2.4.25. Ethylene oxide and dioxan
USP/NF 〈228〉 Ethylene Oxide and Dioxane
USP/NF 〈469〉 Ethylene Glycol, Diethylene Glycol, and Triethylene Glycol in Ethoxylated Substances
And much more
Tests for identity
Ph. Eur. 2.2.24. Absorption Spectrophotometry, Infrared
USP/NF 〈197〉 Spectroscopic Identification Tests
Your individual offer
And that’s just the beginning


Packing Materials Testing
Glass containers
Ph. Eur. 3.2.1
USP/NF 〈660〉
Plastic containers
Ph. Eur. 3.2.3
Ph. Eur. 3.2.4
Ph. Eur. 3.2.5
Ph. Eur. 3.2.6
USP/NF 〈661〉
USP/NF 〈671〉
Rubber Seals / Silicon Elastomers
Ph. Eur. 3.2.9
USP/NF 〈381〉
Ph. Eur. 3.1.9
GMP Testing
GMP Quality Control
Method Development & Validation






Dissolution
Extractables & Leachables






Particle Determination
Elemental Impurities Testing



Regulatory Services
Amino Acid Analysis




N-nitrosamines
qPCR


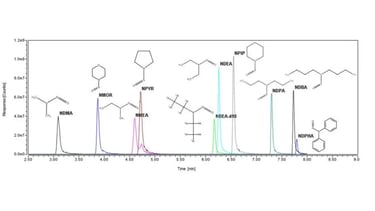


Protein Characterization


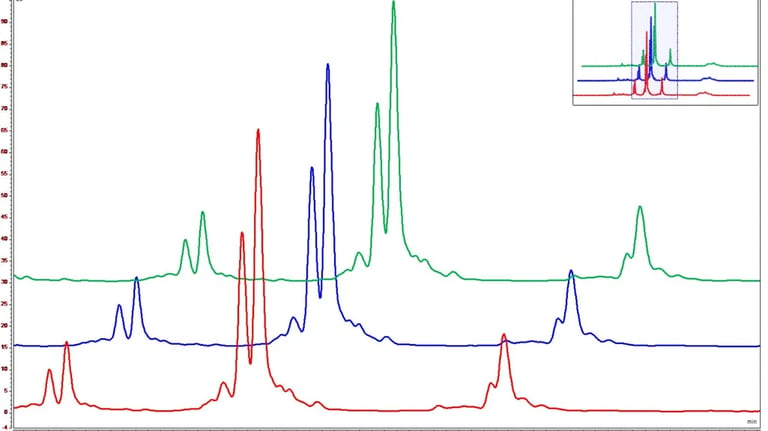
Bioassay
Stability Studies
Packaging Material Testing
Microbiology
Instrumentation
cIEF
Limit test for DEG and EG by GC
Our Services
The experienced and highly committed GBA Pharma Labs team offers a variety of different services in the following Areas.
