Protein characterization
Analytic of proteinogenic drugs
There is a growing need in society for new therapeutic approaches in the form of biopharmaceuticals such as proteinogenic agents, antibody conjugates and immunotherapies for the treatment of serious and rare diseases. For this reason, investments are being made in the rapidly developing field of biologicals with the aim of advancing the development and production of biopharmaceuticals.
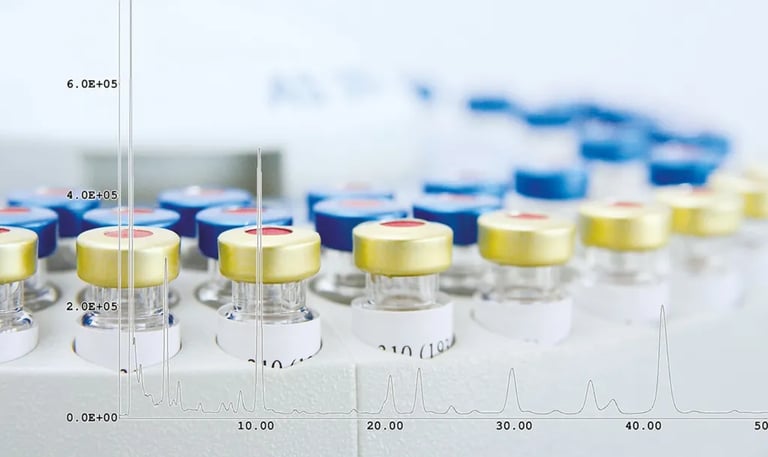
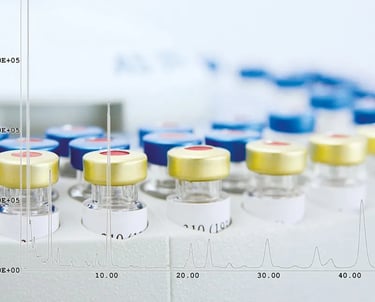
The production and release of biologicals is a challenging process, mainly due to the highly complex protein structures of these molecules. These complex structures require a high level of expertise, both in production and in quality control, which forms the basis for the release of the products. For the latter, specialized primary and secondary reference standards and analytical equipment, particularly in the field of mass spectrometry, are essential in addition to expert knowledge of analytics. The technology in this area is developing rapidly and requires regular investment. GBA Group stays up to date for you and operates an extensive pool of highly specialized analytical equipment. GMP-compliant qualified instruments are available for analysis.
The GBA Group is one of the few laboratories with a GMP qualification for the Orbitrap Exploris from ThermoFisher Scientific. Measurements can be carried out with the GMP and cGMP quality standard.
Protein characterization
The analysis for the quality control of biopharmaceuticals includes as an essential element methods of protein characterization, which are regulated in the European and American pharmacopoeias (Ph.Eur. and USP/NF).
The following topics are to be addressed in detail:
Ph. Eur.:
2.2.43 Mass Spectrometry
2.2.55 Peptide Mapping
2.2.59 Glycan Analysis of Glycoproteins
USP/NF:
<736> Mass Spectrometry
<1736> Applications of Mass Spectrometry
<1055> Biotechnology-Derived Articles – Peptide Mapping
<1084> Glycoprotein and Glycan Analysis – General Considerations
<212> Oligosaccharide Analysis
The characterization of proteins and glycoproteins can take place at different levels:
Level of the intact protein and glycoprotein
Level of the cleaved glycans
Level of peptides and glycopeptides (peptide mapping)
Level of monosaccharides
Level of amino acids
Our Methods
The GBA Group offers a wide range of methods for the analysis of proteinogenic drugs and monoclonal antibodies "for human use":
Analysis at the level of the intact protein and glycoprotein
ESI and APCI mass spectrometry: Orbitrap Exploris from ThermoFisher Scientific
Ion Exchange HPLC / -UHPLC
SEC-HPLC / -UHPLC e.g. determination of aggregates
HIC-HPLC / -UHPLC e.g. for antibody-drug conjugates
Sodium Dodecyl Sulfate – PolyAcrylamid Gel Electrophoresis (SDS-PAGE) e.g. according to Ph. Eur. 2.2.31
native PAGE
Capillary electrophoresis (cGE, CE) e.g. determination of charge variants
Sialylation heterogeneity
Analysis at the glycan level
Enzymatic cleavage using exo-glycosidase
ESI mass spectrometry: Orbitrap Exploris from ThermoFisher Scientific
Anion Exchange - HPLC or UHPLC - MS
HPAEC-PAD chromatography
Fluorophore labeling, e.g. analysis of fluorescently labeled N-glycans using Glycoworks RapiFluor-MS
Capillary electrophoresis of fluorescently labeled glycans
Analysis at the level of peptides and glycopeptides
Peptide mapping is roughly divided into the following steps:
Isolation and purification (isolation of proteins and glycoproteins)
Selective cleavage of the peptide bonds
Chromatographic separation
Identification of peptides:
using ESI mass spectrometry: Orbitrap Exploris from ThermoFisher Scientific e.g. molecular identity determination
with peptide sequencing by ESI-MS-MS: Orbitrap Exploris from ThermoFisher Scientific
by means of HPLC- / UHPLC- UV/VIS
by qualitative and quantitative determination of post-translational modifications such as oxidation methionine
Analyses at the monosaccharide level
HPAEC-PAD chromatography
Fluorophore labeling with RP HPLC
Analyses at the level of amino acids
see "Amino acid analysis according to Ph. Eur. 2.2.56"
GMP Testing
GMP Quality Control
Method Development & Validation






Dissolution
Extractables & Leachables






Particle Determination
Elemental Impurities Testing



Regulatory Services
Amino Acid Analysis




N-nitrosamines
qPCR


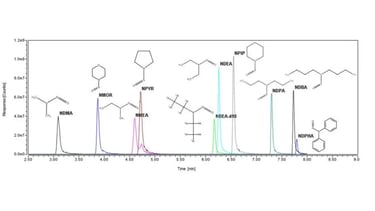


Protein Characterization


Bioassay
Stability Studies
Packaging Material Testing
Microbiology
Instrumentation
cIEF
Limit test for DEG and EG by GC
Our Services
The experienced and highly committed GBA Pharma Labs team offers a variety of different services in the following Areas.


