Instrumentation


Device List and Analysis Methods
If the analysis that you need cannot be found on the list of analytical techniques, then the device list should give an indication of the methods we have established in our labs. For the equipment listed below, we generally have multiple devices available at several locations which ensures that your order will be processed on time. If the device that you are looking for is not on this list, then please contact our marketing department and we will gladly provide you with assistance.
Amino Acid Analyzer:
Amino Acid Analyzer for Ninhydrin-Positive Substances
Bio-Inert LC Systems:
Bio LC-ECD
Bio LC Conductivity Sensor
Biological Methods:
ELISA
Gel Electrophoresis
Western Blot
Chemical-Physical and Pharmaceutical Tests:
Abrasion Resistance
Refractive Index
Hardness
Density
Moisture Determination Using a Glove Box
Freezing Point
Freeze-Drying (Speed Vac)
Gravimetry (Loss on Drying, Total Ash, Residue on Ignition, Sulfated Ash)
Gradation Testing (Sieve Analysis)
Halogen Drying
Conductivity
Microscopy
Wet Chemistry (Ph.Eur, BP, USP, JP)
Osmotic Concentration
Subvisible particles via Particle Counting according Ph.Eur. 2.2.19
Particle Determination (Visible Particles, according to Ph.Eur. 2.9.20)
Particle Size distribution via Laser Light diffraction according Ph.Eur. 2.2.20
Polarimetry
Oxygen Determination
Melting Point
Bulk Density and Tapped Density (incl. Hausner Ratio)
Flash Point
Nitrogen Determination according to Kjeldahl
Tablet Hardness Testing
Titration, Automatic Titrators (e.g. Potentiometric)
Turbidity Measurements
Viscosity Testing (Torque Measurement, Cone/Plate)
Determination of Moisture Content according to Karl-Fischer – Coulometric Ph.Eur.2.5.32
Determination of Moisture Content according to Karl-Fischer – Titration Ph.Eur.2.5.12
Determination of Moisture Content according to Karl-Fischer – Stromboli Oven
Disintegration Testing (Suppositories, Pills)
Elemental Analyses:
AAS (Flame, Graphite Furnace, Hybrid – Batch and FIA Techniques)
ICP-MS
ICP-OES
Active Ingredient Dissolution:
Dissolution Testing using flow-through cells according to USP-IV for Active Ingredients with Low Solubility, Granules, Suppositories, Soft Gelatin Capsules
Dissolution Testing according to USP-I & USP-II; Offline and Online UV Detection
GC Systems:
Headspace-GC-FID
Headspace-GC-MS
GC-ECD
GC-FID
GC-MS
GC-PND
GC-WLD
GC-PTV Injection System
GC-Split/Splitless
HPTLC/TLC:
HPTLC/TLC Qualitative Analysis with Photo Documentation (UV, VIS, Fluorescence)
HPTLC Quantitative
LC Systems:
HPLC-CAD
HPLC-DAD
HPLC-ECD
HPLC-ELSD
HPLC-Fluorescence
HPLC-Conductivity Detector
HPLC-MS
HPLC-MS-QTOF
HPLC-PAD
HPLC-RI
HPLC-UV/VIS
HPLC-Post-Column Derivatization
GPC
GPC-RI (Molar Mass Distribution)
Ion Chromatography (Anions, Cations, Ion Suppression)
UPLC-DAD
UPLC-MS
UPLC-UV
Capillary Electrophoresis
Mass Spectrometry:
GC-MS
HPLC-MS
HPLC-MS-QTOF
UPLC-MS
Spectrophotometry:
FT-IR (ATR)
UV/VIS Spectrophotometer
Stability Studies:
Temperature-Controlled Stability Rooms and Chambers (all ICH Conditions; Special Conditions from -80°C to +55°C)
Photostability Tests: Atlas Suntester, WUT Stability Chamber (ICH Q1B)
GMP Testing
GMP Quality Control
Method Development & Validation






Dissolution
Extractables & Leachables






Particle Determination
Elemental Impurities Testing



Regulatory Services
Amino Acid Analysis




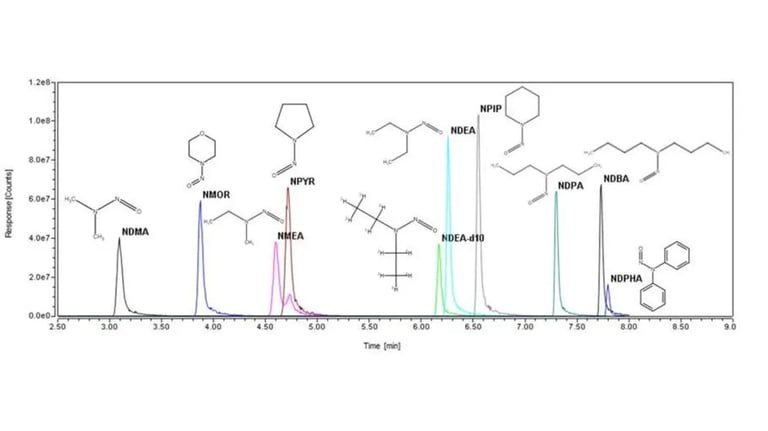
N-nitrosamines
qPCR


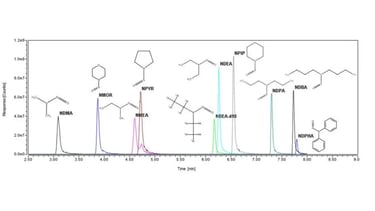


Protein Characterization


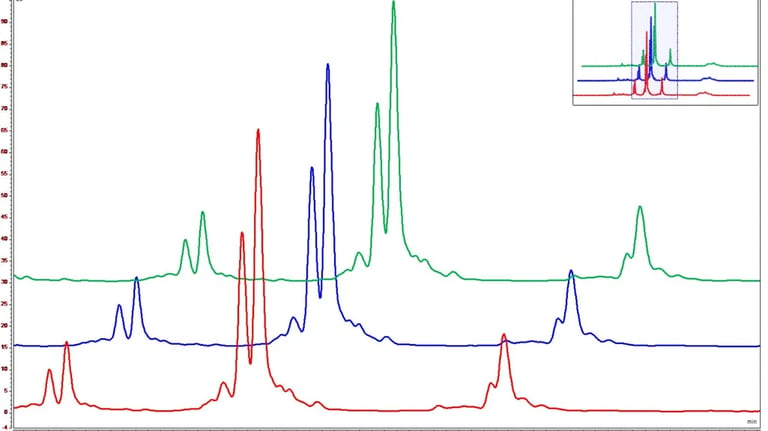
Bioassay
Stability Studies
Packaging Material Testing
Microbiology
Instrumentation
cIEF
Limit test for DEG and EG by GC
Our Services
The experienced and highly committed GBA Pharma Labs team offers a variety of different services in the following Areas.


