cIEF (Capillary Isoelectric Focusing)


A Cutting-Edge Technique for Quality Control in the Pharma Industry
In the pharmaceutical industry, ensuring the purity and stability of biopharmaceutical products is of utmost importance. To meet these requirements, the industry has embraced advanced analytical techniques, with cIEF emerging as a powerful tool for quality control. cIEF offers high-resolution separation and precise characterization of proteins, enabling pharmaceutical companies to assess product quality with unparalleled accuracy.
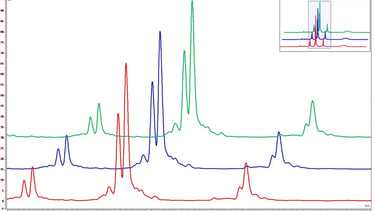
Protein Separation and Analysis: cIEF is a technique that separates proteins based on their pI (isoelectric point), a property that reflects their overall charge at a specific pH. By employing a capillary filled with a pH gradient, proteins migrate towards their isoelectric point and separate based on their charge differences. This high-resolution separation allows for the detection of impurities, variants, or modifications in biopharmaceutical products, ensuring product purity and consistency.
Identification of Protein Variants and Modifications: Biopharmaceutical products often undergo post-translational modifications or may contain protein variants due to production processes. cIEF enables the precise identification and characterization of these variants and modifications. By comparing the migration patterns of reference proteins, the presence of charge variants or modifications, such as glycosylation or deamidation, can be detected. This information is crucial for ensuring product stability, efficacy, and safety.
Quantification and Batch Consistency: cIEF not only separates proteins but also allows for their quantification. By using appropriate calibration standards, the concentration of target proteins can be determined accurately. This information aids in assessing batch-to-batch consistency, verifying product specifications, and ensuring reproducibility. The ability to quantify proteins in complex matrices contributes to the overall assessment of product quality and the maintenance of manufacturing standards.
Detection of Impurities and Contaminants: cIEF serves as a powerful tool for detecting impurities and contaminants in biopharmaceutical products. It can identify trace amounts of host cell proteins, residual DNA, aggregates, or other impurities that may impact product safety and efficacy. The sensitivity of cIEF enables the detection and quantification of these impurities, supporting the stringent quality control requirements of the pharmaceutical industry.
Conclusion: cIEF has revolutionized quality control in the pharmaceutical industry by providing high-resolution separation and precise characterization of proteins. Its ability to identify protein variants, modifications, quantify target proteins, and detect impurities makes it an indispensable tool for assessing the purity, stability, and consistency of biopharmaceutical products. By leveraging the power of cIEF, pharmaceutical companies can ensure the highest standards of quality, ultimately leading to the delivery of safe and effective medications to patients worldwide.
GMP Testing
GMP Quality Control
Method Development & Validation






Dissolution
Extractables & Leachables






Particle Determination
Elemental Impurities Testing



Regulatory Services
Amino Acid Analysis




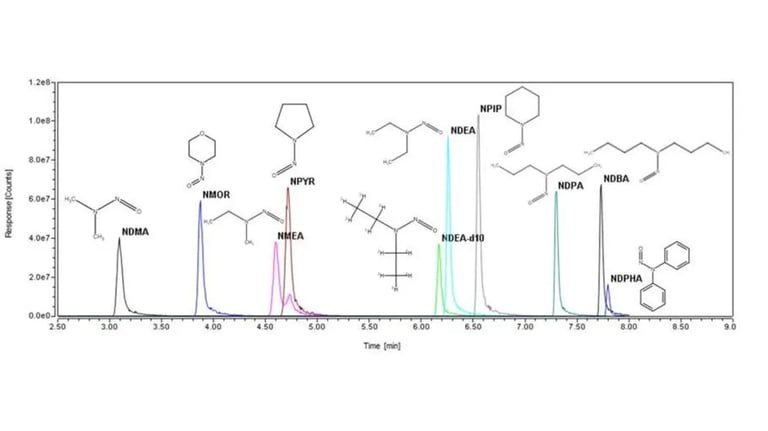
N-nitrosamines
qPCR


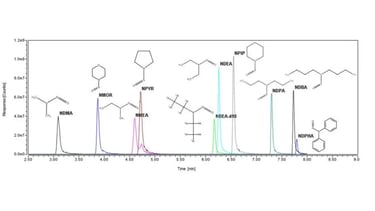


Protein Characterization


Bioassay
Stability Studies
Packaging Material Testing
Microbiology
Instrumentation
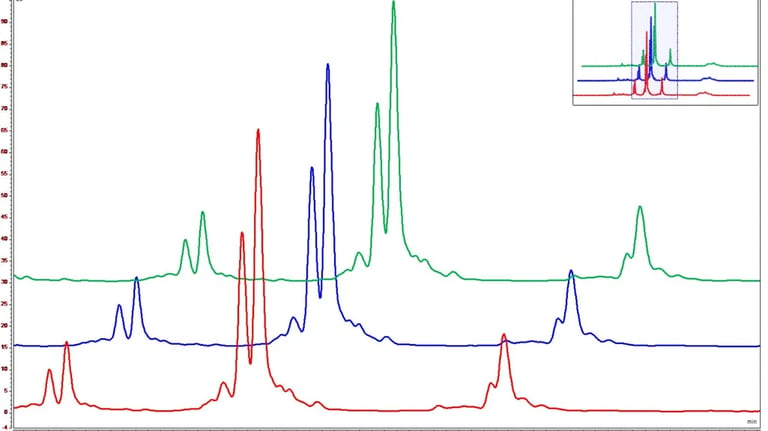
cIEF
Limit test for DEG and EG by GC
Our Services
The experienced and highly committed GBA Pharma Labs team offers a variety of different services in the following Areas.


