qPCR Analysis


A Precise Tool for Quality Control in the Pharma Industry
In the pharmaceutical industry, maintaining stringent quality control measures is essential to ensure the safety and efficacy of medications. To achieve this, the industry has adopted innovative techniques, with qPCR (quantitative Polymerase Chain Reaction) analysis emerging as a powerful tool. qPCR has revolutionized quality control processes, offering enhanced precision, sensitivity and accuracy.
Accurate Quantification:
qPCR analysis enables the precise quantification of nucleic acids, making it invaluable in pharmaceutical quality control. By utilizing fluorescently-labeled probes or DNA-binding dyes, qPCR allows for the real-time monitoring of the amplification process. This real-time aspect enables the quantification of target DNA or RNA sequences accurately, providing critical information about the presence and quantity of contaminants or impurities in pharmaceutical products.
Detection of Low Abundance Targets:
In the pharma industry, it is crucial to identify and measure low abundance targets, such as residual host cell DNA or RNA, which may impact product safety and efficacy (Ph.Eur. 2.6.35 Quantification and characterisation of resibual host-cell DNA) oder Mykoplasmen (Ph.Eur. 2.6.7 Mycoplasmas, NAT).
qPCR analysis excels in detecting and quantifying these targets due to its high sensitivity. By utilizing specific primers and probes, qPCR can selectively amplify and detect even trace amounts of target sequences, ensuring the precise measurement of impurities in pharmaceutical products.
Rapid and High-Throughput Analysis:
Time is of the essence in pharmaceutical quality control, and qPCR analysis offers rapid and high-throughput capabilities. Compared to traditional PCR methods, qPCR significantly reduces analysis time. Multiple samples can be processed simultaneously, thanks to automation and the use of microtiter plates. This efficient workflow allows pharmaceutical companies to perform large-scale quality control testing with speed and accuracy.
Validation of Process Efficiency:
qPCR analysis provides insights into process efficiency and validation in pharmaceutical manufacturing. By monitoring the expression of specific genes or the presence of genetic markers, qPCR can assess the consistency and reproducibility of manufacturing processes. This information helps identify any deviations or variations, enabling prompt corrective actions to maintain product quality and reliability.
Conclusion:
qPCR analysis has become an indispensable tool in pharmaceutical quality control, providing accurate detection and quantification of low abundance targets, rapid analysis, and process efficiency validation. With its ability to deliver reliable and precise results, qPCR empowers the industry to uphold the highest standards of quality, ensuring the safety and efficacy of pharmaceutical products. As technology continues to advance, the future of qPCR holds immense potential for further enhancing quality control measures within the pharmaceutical industry.
GMP Testing
GMP Quality Control
Method Development & Validation






Dissolution
Extractables & Leachables






Particle Determination
Elemental Impurities Testing



Regulatory Services
Amino Acid Analysis




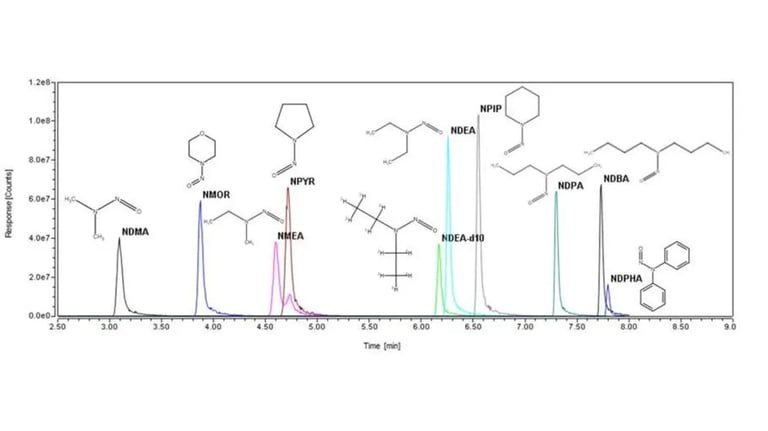
N-nitrosamines
qPCR


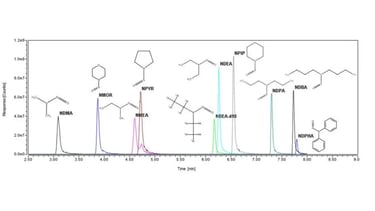


Protein Characterization


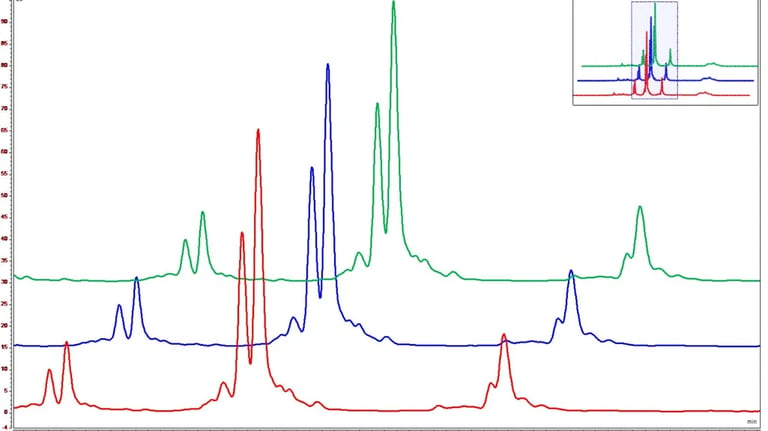
Bioassay
Stability Studies
Packaging Material Testing
Microbiology
Instrumentation
cIEF
Limit test for DEG and EG by GC
Our Services
The experienced and highly committed GBA Pharma Labs team offers a variety of different services in the following Areas.


