Microbiology and Molecular Biology


Microbiology
New Services (cGMP)
Performing Chromogenic Assays (e.g. Heparin according to Ph.Eur. 2.7.5 and USP <208>; ELISA for Penicillins)
Endotoxin Testing according to Ph.Eur. 2.6.14 (Methods C, D) and USP <85> using Turbidimetric and Chromogenic Methods
Microbiological examination of nonsterile products: Tests for Burholderia cepacia complex in accordance USP<60>
TOC (total organic carbon) in accordance Ph. Eur. 2.2.44, USP <643>, JP und KP
Tests in accordance with Ph.Eur. (cGMP)
Microbiological Examination of Non-Sterile Products: Microbial Enumeration Tests acc. to 2.6.12
Microbiological Examination of Non-Sterile Products: Test for Specified Microorganisms acc. to 2.6.13
Microbial Examination of Herbal Medicinal Products for Oral Use and Extracts Used in Their Preparations acc. to 2.6.31
Bacterial Endotoxins acc. to 2.6.14 (Method A)
Microbiological Assay of Antibiotics acc. to 2.7.2
Efficacy of Antimicrobial Preservation acc. to 5.1.3
Water, Purified (Aqua purificata; Monograph 0008)
Water for Injection (Monograph 0169)
Water for Preparation of Extracts (Monograph 2249)
Tests in accordance with USP (cGMP)
Chapter <51> Antimicrobial Effectiveness Testing
Chapter <61> Microbiological Examination of Non-Sterile Products: Microbial Enumeration Test
Chapter <62> Microbiological Examination of Non-Sterile Products: Test for Specified Microorganisms
Chapter <81> Antibiotics-Microbial Assay
Chapter <85> Bacterial Endotoxins Test
Additional Services
Product-Specific Validations (acc. to Ph.Eur. and USP)
Development and Validation of Rapid Test Methods (e.g. Simplate®)
Microbial Assay of Vitamins
Identification of Germs
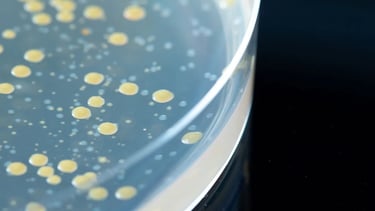
Microbiological Tests - Hygiene Monitoring (cGMP)
Sampling by Trained Stuff
Investigation
Germ Identification to Genus Level (Identification on a Species Level can be done via Subcontracting)
On request we can supply the necessary additional information about detected bacteria and their possible sources of contamination
Molecular BiologyCell-based tests / biological activity tests
Cell migration assays
Cell proliferation/inhibition assays
Binding and/or competitive assays
Monocyte activation test (Ph. Eur. 2.6.30)
Enzyme-linked Immunosorbent Assay (ELISA)
Residual Host Cell Protein Assays (HCP) with ELISA (e.g. according to Ph. Eur. 2.6.34)
RNAse/DNAse through Gel electrophoresis
GMP Testing
GMP Quality Control
Method Development & Validation






Dissolution
Extractables & Leachables






Particle Determination
Elemental Impurities Testing



Regulatory Services
Amino Acid Analysis




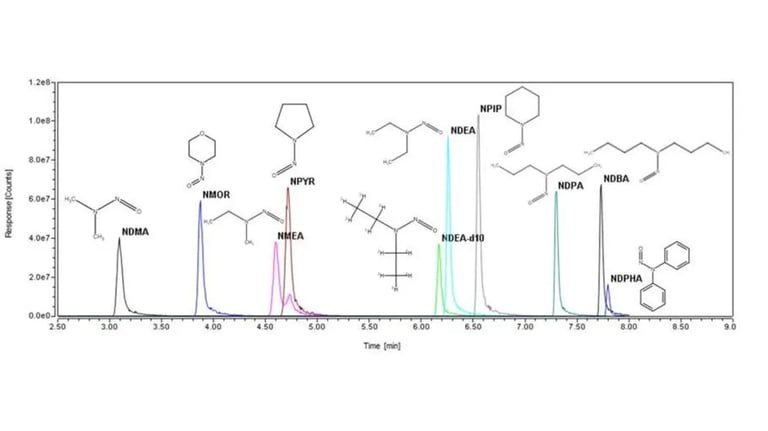
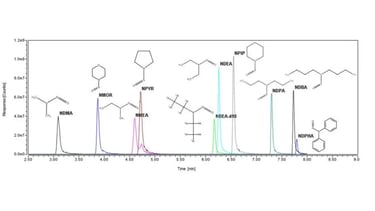
N-nitrosamines
qPCR




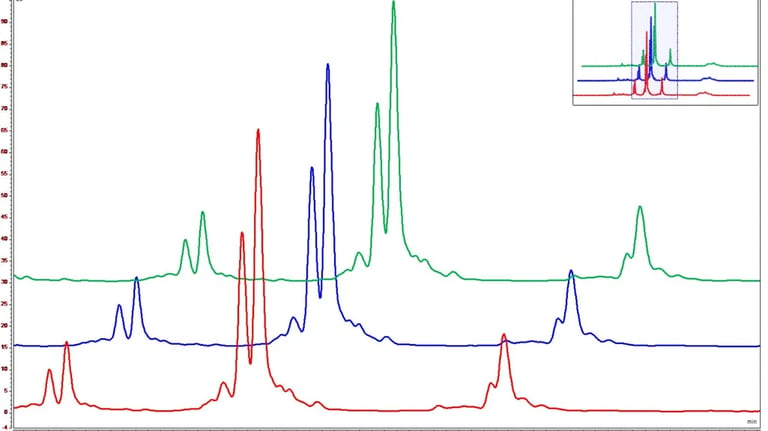
Protein Characterization


Bioassay
Stability Studies
Packaging Material Testing
Microbiology
Instrumentation
cIEF
Limit test for DEG and EG by GC
Our Services
The experienced and highly committed GBA Pharma Labs team offers a variety of different services in the following Areas.


