Asbestos in Talcum


USP latest update on Talcum
The USP Monograph Talc, which introduces stricter testing requirements for asbestos in talc using advanced methods: light microscopy and x-ray diffractometry, was implemented and is effective of December 1st 2025. For the first time the monograph mandates the use of both Light Microscopy (LIM) and X-Ray Diffraction (XRD).
These changes intend to ensure the highest safety and quality standards for talc-containing products.
GBA IS READY TO SUPPORT YOUR ANALYTICAL NEEDS UNDER GMP IN OUR FDA INSPECTED LABORATORY TO ENSURE THE REGULATORY COMPLIANCE OF YOUR MATERIALS AND PRODUCTS IN ACCORDANCE WITH THIS NEW MANDATORY REQUIREMENT.
Analysis can be performed directly under GMP due to our in-house validated method, cutting analytical costs and significantly reducing reporting time.
Contact: Vanya Koteva
Head of Sales & Business Development
Tel. +49 173 889 42 51
Email: sales@gba-pharma.com
GMP Testing
GMP Quality Control
Method Development & Validation






Dissolution
Extractables & Leachables






Particle Determination
Elemental Impurities Testing

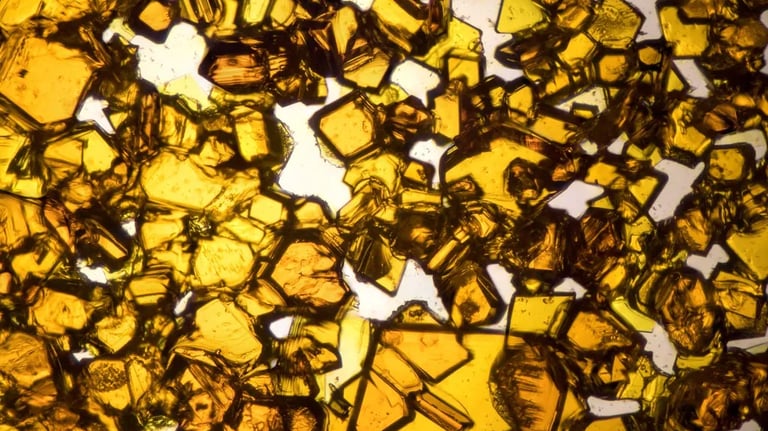


Regulatory Services
Amino Acid Analysis




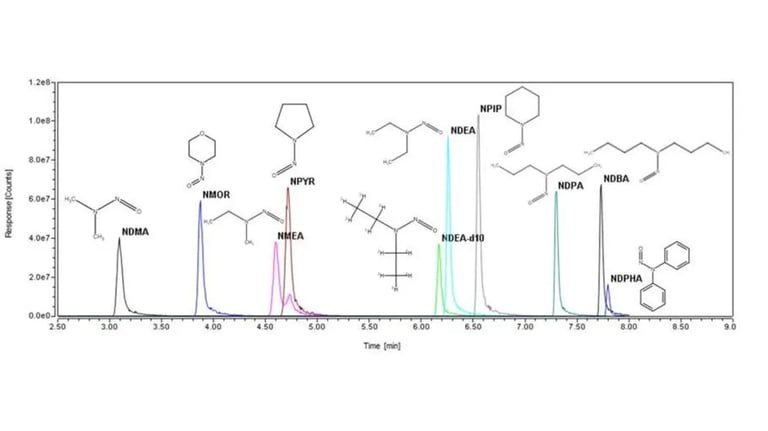
N-nitrosamines
qPCR


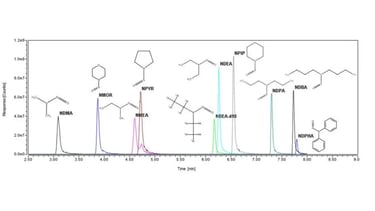


Protein Characterization


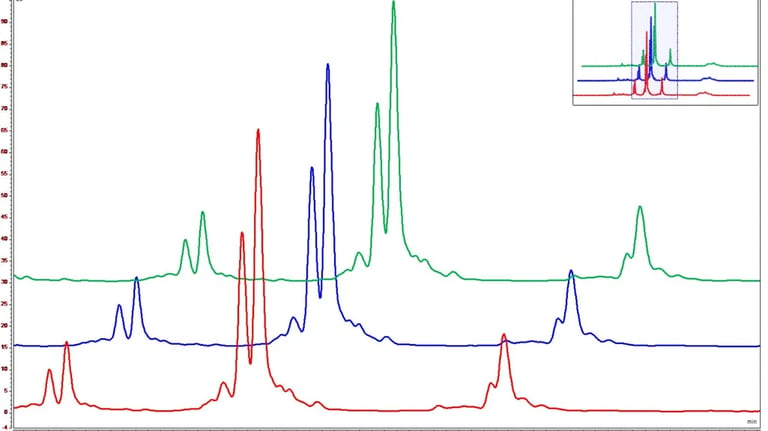
Bioassay
Stability Studies
Packaging Material Testing
Microbiology
Instrumentation
cIEF
Limit test for DEG and EG by GC
Our Services
The experienced and highly committed GBA Pharma Labs team offers a variety of different services in the following Areas.


