Stability Studies


Comprehensive service
In the field of stability testing, we offer a comprehensive service for human and veterinary medicinal products as well as for medical devices.
Our service includes consulting and planning of your stability studies as well as all necessary testing and quality assessments.
Storage of your samples under the required conditions (e.g. ICH conditions and special conditions)
Stability study sample management
Perform the pre-planned analyses on stability samples as scheduled.
Our Services in Detail:
Preparation and forwarding of storage protocols (storage summary) after recording the samples in the LIMS - Transparency regarding storage and retrieval times
Storage of your samples as part of stability studies with GMP monitoring
o Technical studies
o Development studies
o Follow-up
o ICH-studies
o On-going studies
o Simulation of in-use StudienStability protocol
o Consulting and Assistance in the Planning of Stability Studies
o Implementation and realisation of submitted stability plansOutsourcing at the agreed time
Subsequent analyses and submission of results
Automatic shipping of stability samples
Transport studies / freeze-thaw / cycle test
Stress tests Stability indicators
o Photostability testing
o Thermal stress
o Acid or alkaline stress
o Oxidative or reductive stressingInterim storage in case of capacity bottlenecks and as a backup if your chambers fail
Storage and shipping of samples possible without analytical services
Your stability samples can be stored under various climatic conditions:
As standard, we store in the following climatic conditions:
Long-Term: 25 °C / 60 % r.h.
Intermediate: 30 °C / 65 % r.h.
Accelerated: 40 °C / 75 % r.h.
Fridge/Freezer: 5 °C / -20 °C / -80 °C
Desired storage condition not included? We are happy to check the feasibility on our variably adjustable devices.
Your samples are in good hands with us. The storage conditions are monitored 24/7. We have decades of experience in performing stability studies.
Online monitoring
Automated alerting
LIMS module for stability studies with automated reminder to pull samples
Staff specifically trained to manage stability studies
Restricted access to stability chambers for trained personnel only
Storage capacity
approx. 300 m³
PhotostabilityWe offer the test for photostability
In addition to our comprehensive services regarding stability testing and storage, we offer the test for photostability according to guideline ICH Q1B "Stability Testing: Photostability Testing of New Drug Substances and Products".
Equipment, Methods, Procedures
EquipmentTemperature- and Moisture-Regulated Photostability Chambers
The Endpoint Detection is Performed with Calibrated Sensors
We analyzeDrugs
Finished Pharmaceutical Preparations
Primary Packed Products
Cosmetics
AnalysisAnalysis of the Unexposed and the Exposed Sample
Analysis of Reference Samples
Determination the Physical Properties - Assay
Detection and Analysis of Degradation Products
Dissolution / Drug release
DoseUV (300-400 nm): At least 100 Wh/m²
VIS (400-800 nm): At least 1.2 million lx
The light intensity and temperature during the tests are measured on the surface of the sample.
GMP Testing
GMP Quality Control
Method Development & Validation






Dissolution
Extractables & Leachables






Particle Determination
Elemental Impurities Testing



Regulatory Services
Amino Acid Analysis




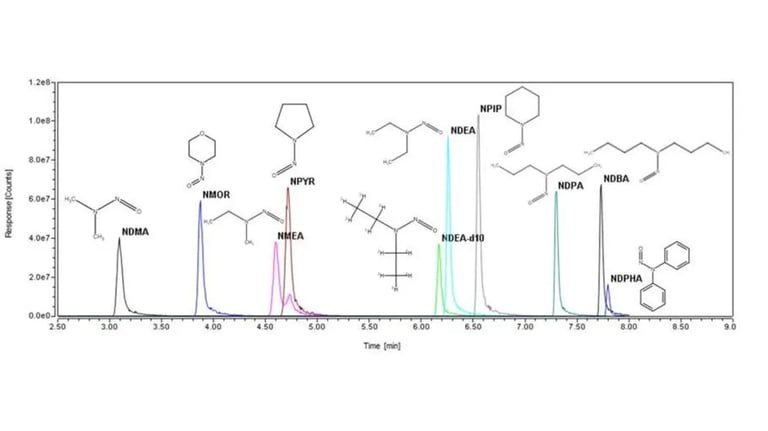
N-nitrosamines
qPCR


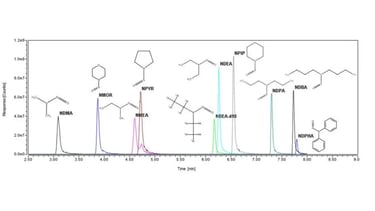


Protein Characterization


Bioassay
Stability Studies
Packaging Material Testing
Microbiology
Instrumentation
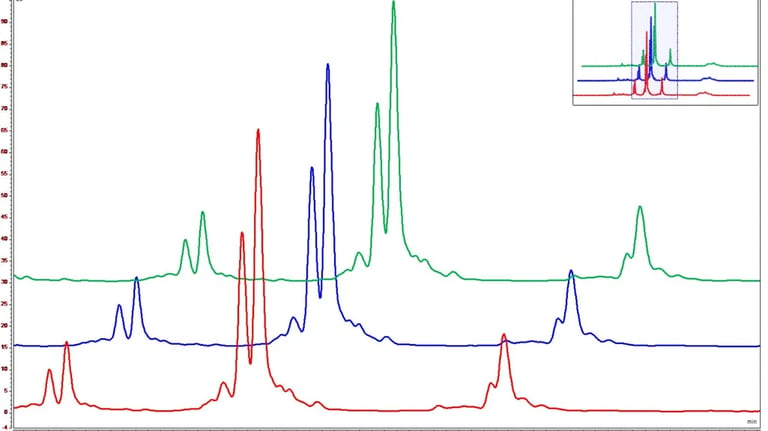
cIEF
Limit test for DEG and EG by GC
Our Services
The experienced and highly committed GBA Pharma Labs team offers a variety of different services in the following Areas.


