Packaging Material Testing


Testing the quality of a medicinal product
Testing the quality of a medicinal product includes more than just analyzing the ingredients. It is becoming increasingly important to test pharmaceutical packaging materials, mainly the primary containers, which come in direct contact with the product. The regulatory demands in this field have increased significantly in recent years.
The guidelines include testing the raw materials used, the finished packaging, as well as the seals of the packaging.
The GBA Group can support you in the following areas:
Testing Glass Containers according to Ph.Eur. 3.2.1 and USP <660>
Testing Plastic Materials and Containers (e.g. PE, PP, PET) according to Ph.Eur. 3.2.3 – 3.2.6, USP <661>, <671>
Testing Rubber Seals according to Ph.Eur. 3.2.9 and USP <381>
Testing Silicon Elastomers according to Ph.Eur. 3.1.9
Extractables & Leachables Studies
Integrity tests for container closures:
Container Closure Integrity Tests (CCIT) according to USP <1207>
Tracer Liquid Test
Microbial Challenge Test (microbiological testing)
GMP Testing
GMP Quality Control
Method Development & Validation






Dissolution
Extractables & Leachables






Particle Determination
Elemental Impurities Testing



Regulatory Services
Amino Acid Analysis




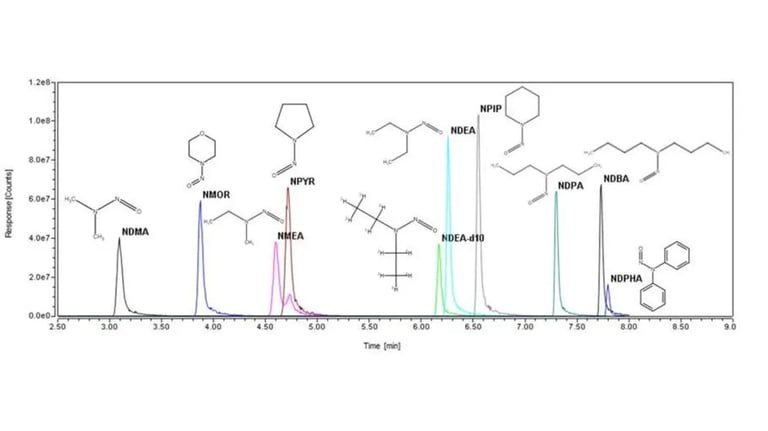
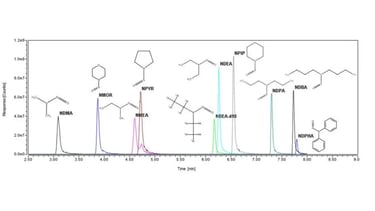
N-nitrosamines
qPCR




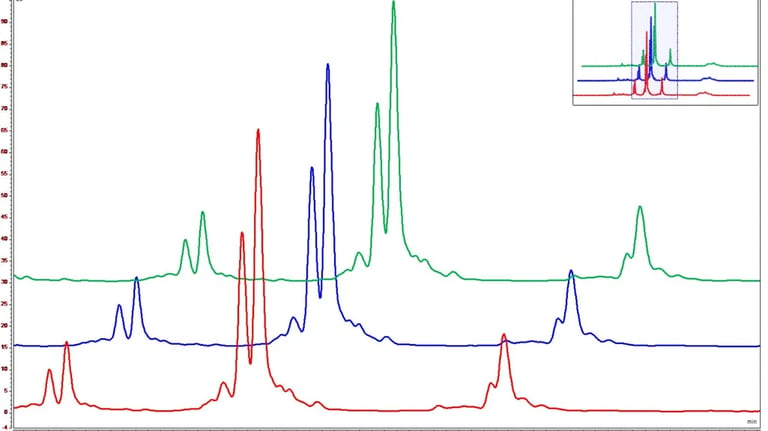
Protein Characterization


Bioassay
Stability Studies
Packaging Material Testing
Microbiology
Instrumentation
cIEF
Limit test for DEG and EG by GC
Our Services
The experienced and highly committed GBA Pharma Labs team offers a variety of different services in the following Areas.


