Method Development & Validation
Method Development & Validation
A lot of facts have to be considered
The development and validation of a new method often seems to be hard and complex. Prior to the start, a lot of facts have to be considered. Beneath the availability of qualified personal the performance of the new method has to meet internal and general quality guidelines and should be suitable for everyday use. Especially with biotherapeutics, you are forced to follow new analytical paths, as new synthetic pathways are explored continuously.
Save time, money and own resources, which might be used more efficiently elsewhere. Take advantage of the know-how and the capacity of the GBA Group.
In the field of human and veterinary drugs, biologic therapies and herbal medicines we offer.
Development and Validation of New Analytical Methods for:
APIs and Raw Materials used for Pharmaceutics and Biotherapeutics
Chemically Defined Substances
Medical Devices
Development and Validation of Impurity Testing
Degradation Products in Finished Formulations
By-Products Resulting from Synthesis of Active Substances made for Pharmaceutics and Biotherapeutics
Trace Components and Detergents (Tween, Pluronic, Triton, etc.)
Forced degradation studies
GMP Testing
GMP Quality Control
Method Development & Validation






Dissolution
Extractables & Leachables






Particle Determination
Elemental Impurities Testing



Regulatory Services
Amino Acid Analysis




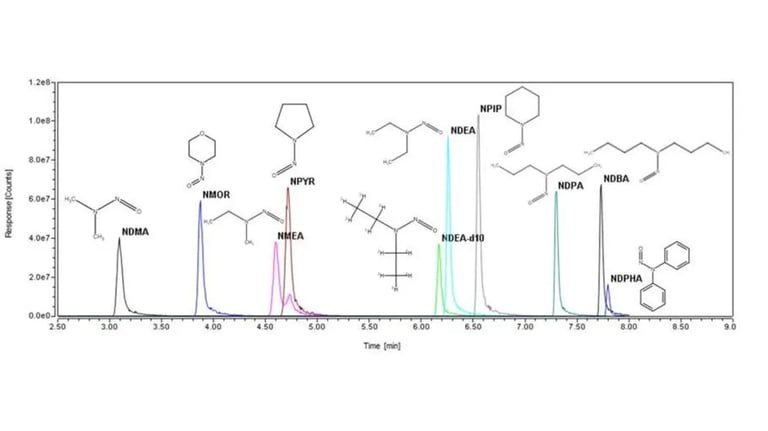
N-nitrosamines
qPCR


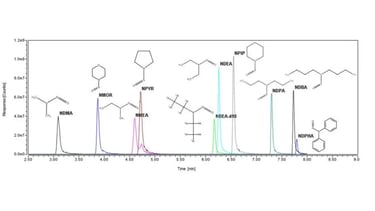


Protein Characterization


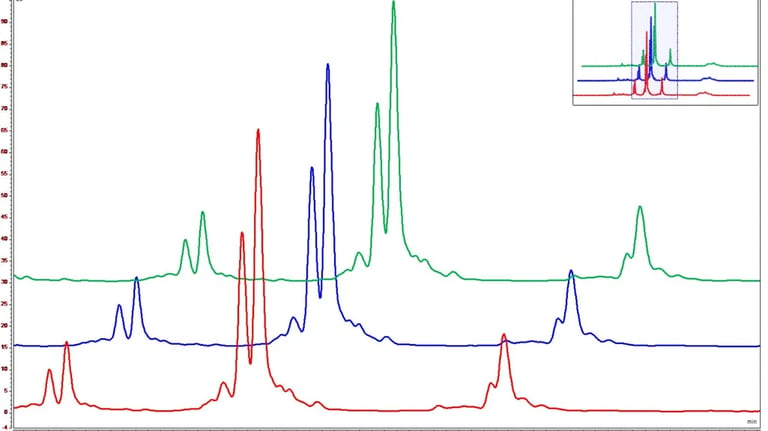
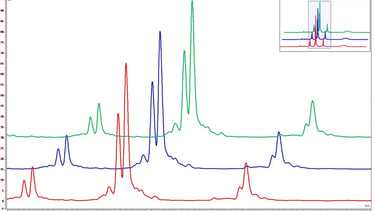
Bioassay
Stability Studies
Packaging Material Testing
Microbiology
Instrumentation
cIEF
Limit test for DEG and EG by GC
Our Services
The experienced and highly committed GBA Pharma Labs team offers a variety of different services in the following Areas.


