Our Services
The experienced and highly committed GBA Pharma Labs team offers a variety of different services in the following Areas.

GMP Testing
The GBA Pharma Labs runs two GMP-certified and
FDA-inspected sites in Germany with more than 170 highly qualified employees and over 7500 m² of lab space. These subsidiaries can draw upon decades of experience in pharmaceutical analysis and assisting with the pharmaceutical approval process in human and veterinary medicine. Over this time, they have built up a global customer base by providing reliable and faithful cooperation.
Our laboratories are certified according to EU-GMP, and US(FDA)-GMP, and we have a manufacturing license based on §13 (1) AMG (German pharmaceutical law) as well as an import license based on §72 AMG.
GMP Quality Control
One focus of our activity is the testing of drugs, biotherapeutics, and medical devices in accordance with the methods of pharmacopeia. We also offer the testing of all required raw materials for production, such as active ingredients, excipients, and packaging materials. We offer a quick, reliable and prompt service. With modern, comprehensive equipment and the experience of over twenty years in performing analytical analysis, we are able to provide sophisticated and efficient solutions at any time. Right from the preparation of the testing sample and its analysis up to the compilation of the related documentation.
Testing of Active Ingredients and Excipients (Analytics in accordance with the methods of the relevant pharmacopoeia (Ph.Eur., USP, BP, JP, CP)
Testing of Finished Products in the Field of Human and Veterinary Pharmaceuticals, Biotherapeutics, Medical Devices (Testing according to pharmacopoeia or customer method)
Testing of Packaging Materials
Implementation of Analytical Methods including Transfer, Monitoring and Reporting
Testing of Semi-Finished Goods
Testing of Small Scale Batches and Batches from Development (from phase 1 to market)


Method Development & Validation
The development and validation of a new method often seems to be hard and complex. Prior to the start, a lot of facts have to be considered. Beneath the availability of qualified personal the performance of the new method has to meet internal and general quality guidelines and should be suitable for everyday use. Especially with biotherapeutics, you are forced to follow new analytical paths, as new synthetic pathways are explored continuously.
Save time, money and own resources, which might be used more efficiently elsewhere. Take advantage of the know-how and the capacity of the GBA Group.
In the field of human and veterinary drugs, biologic therapies and herbal medicines we offer.
Development and Validation of New Analytical Methods for:
APIs and Raw Materials used for Pharmaceutics and Biotherapeutics
Chemically Defined Substances
Medical Devices
Development and Validation of Impurity Testing
Degradation Products in Finished Formulations
By-Products Resulting from Synthesis of Active Substances made for Pharmaceutics and Biotherapeutics
Trace Components and Detergents (Tween, Pluronic, Triton, etc.)
Forced degradation studies
Stability Studies
In the field of stability testing, we offer a comprehensive service for human and veterinary medicinal products as well as for medical devices.
Our service includes consulting and planning of your stability studies as well as all necessary testing and quality assessments.
Storage of your samples under the required conditions (e.g. ICH conditions and special conditions)
Stability study sample management
Perform the pre-planned analyses on stability samples as scheduled
Our Services in Detail:
Preparation and forwarding of storage protocols (storage summary) after recording the samples in the LIMS - Transparency regarding storage and retrieval times
Storage of your samples as part of stability studies with GMP monitoring
Technical studies
Development studies
Follow-up
ICH-studies
On-going studies
Simulation of in-use Studien
Stability protocol
Consulting and Assistance in the Planning of Stability Studies
Implementation and realisation of submitted stability plans
Outsourcing at the agreed time
Subsequent analyses and submission of results
Automatic shipping of stability samples
Transport studies / freeze-thaw / cycle test
Stress tests Stability indicators
Photostability testing
Thermal stress
Acid or alkaline stress
Oxidative or reductive stressing
Interim storage in case of capacity bottlenecks and as a backup if your chambers failtorage and shipping of samples possible without analytical services
The determination of dissolution rate is an essential part of quality control. Dissolution testing aims to determine the rate at which an active pharmaceutical ingredient (API) is released from its formulation under standardized conditions. In practice, the quantity of drug dissolved in the dissolution medium at time X after addition of the FDF or API is determined. The amount of time required to dissolve the desired amount of API can vary significantly depending on the product being tested.
In the case of immediate release dosage forms, the test is usually carried out at a single point in time (usually 45 minutes or less). Multiple samples are collected in the case of extended or delayed release.
So-called dissolving profiles are also possible. For this purpose, the active substance content in the dissolution medium is determined at several time points and displayed as a function of time.
The results obtained can be used to indicate batch-to-batch consistency and consistent efficacy of the product. In addition, batch homogeneity, compliance and stability can be tested.
The dissolution rate is usually measured for the following dosage forms:
Solid dosage forms (e. g. capsules, tablets, medicated chewing gums)
Pure solid susbstances / active substances in preparations presented as powders or granules (apparent dissolution rate)
Lipophilic dosage forms (e. g. suppositories, soft capsules)
Transdermal patches
The GBA Group is experienced in testing all of the following dosage forms.
Dissolution Test




Our goal is to support manufacturers
Our goal is to support manufacturers of pharmaceutics to select the best primary packaging material for their products.
Primary packaging materials for products such as injectables, inhalers, and generally liquid and topical preparations are made of different materials. Extractables and Leachables (E&L) studies allow to analyse the impact of primary packaging material on the product and determine criteria for a selection and processing, which ultimately serve one purpose: to provide maximum patient protection.
Unfortunately, binding regulatory requirements on how to perform E&L studies are still missing and affected producers are often uncertain about the necessary efforts, costs and testing procedures.
In the field of E&L we have the experience, the know-how and the necessary equipment to offer you a comprehensive guidance when selecting the primary package material or planning E&L offer studies.
Our Services in Detail
Review and Optimization of the Packaging System
Analysis of the Production Process
Risk Assessment of Product Quality
Optimizing of the Manufacturing and Packaging Process
Characterizing and Selecting Suitable Packaging Materials for Maximum Product Protection
Examining Possible Interactions between Packaging Components and Product
Conducting Extraction Studies and Analysis with Coupled Techniques such as GC/MS and Headspace-GC/MS, LC-MS, ICP-MS
Extractables & Leachables



GMP Testing
GMP Quality Control
Method Development & Validation






Dissolution
Extractables & Leachables






Particle Determination
Elemental Impurities Testing



Regulatory Services
Amino Acid Analysis




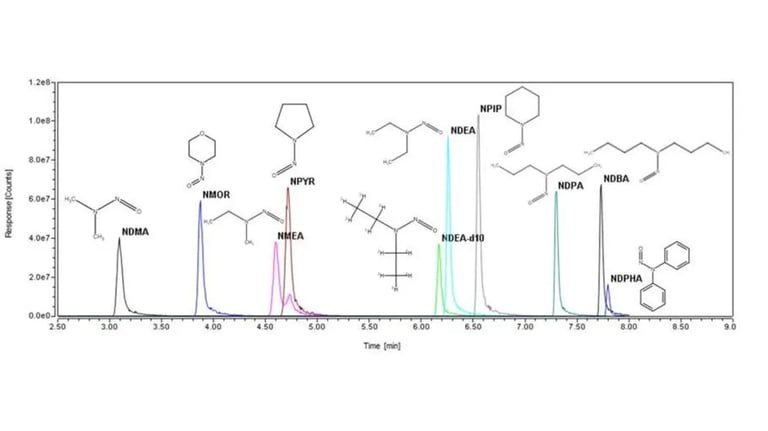
N-nitrosamines
qPCR


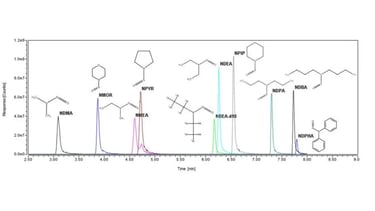


Protein Characterization


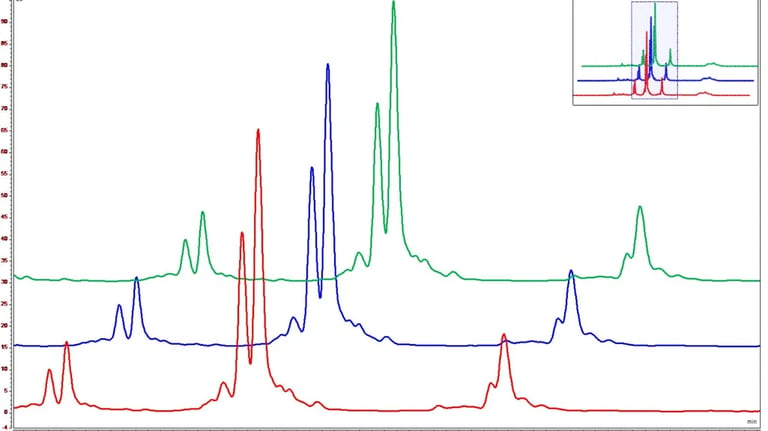
Bioassay
Stability Studies
Packaging Material Testing
Microbiology
Instrumentation
cIEF
Limit test for DEG and EG by GC
Our Services
The experienced and highly committed GBA Pharma Labs team offers a variety of different services in the following Areas.
