Extractables & Leachables
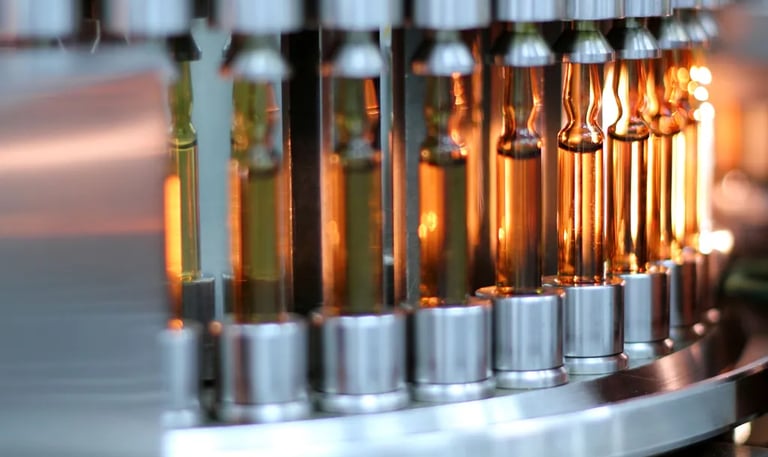
Our goal is to support manufacturers
Our goal is to support manufacturers of pharmaceutics to select the best primary packaging material for their products.
Primary packaging materials for products such as injectables, inhalers, and generally liquid and topical preparations are made of different materials. Extractables and Leachables (E&L) studies allow to analyse the impact of primary packaging material on the product and determine criteria for a selection and processing, which ultimately serve one purpose: to provide maximum patient protection.
Unfortunately, binding regulatory requirements on how to perform E&L studies are still missing and affected producers are often uncertain about the necessary efforts, costs and testing procedures.
In the field of E&L we have the experience, the know-how and the necessary equipment to offer you a comprehensive guidance when selecting the primary package material or planning E&L offer studies.
Our Services in Detail
Review and Optimization of the Packaging System
Analysis of the Production Process
Risk Assessment of Product Quality
Optimizing of the Manufacturing and Packaging Process
Characterizing and Selecting Suitable Packaging Materials for Maximum Product Protection
Examining Possible Interactions between Packaging Components and Product
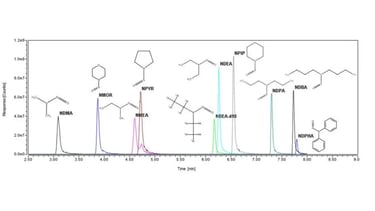
Conducting Extraction Studies and Analysis with Coupled Techniques such as GC/MS and Headspace-GC/MS, LC-MS, ICP-MS
GMP Testing
GMP Quality Control
Method Development & Validation






Dissolution
Extractables & Leachables






Particle Determination
Elemental Impurities Testing



Regulatory Services
Amino Acid Analysis




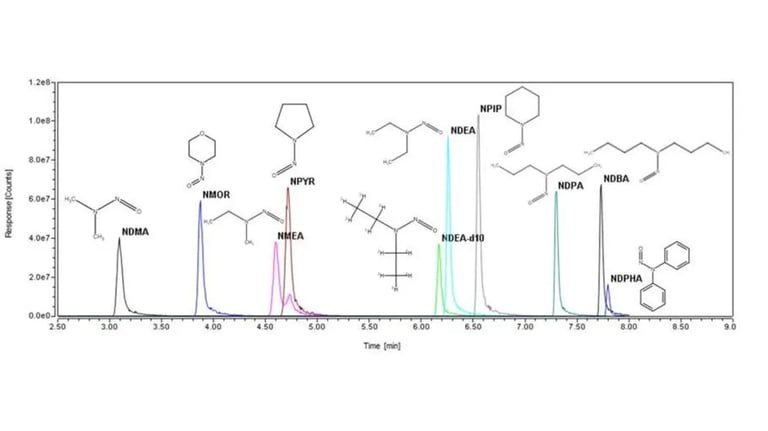
N-nitrosamines
qPCR




Protein Characterization


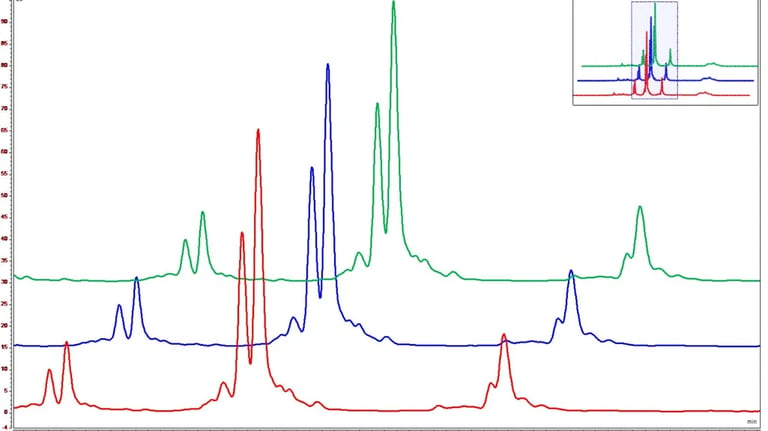
Bioassay
Stability Studies
Packaging Material Testing
Microbiology
Instrumentation
cIEF
Limit test for DEG and EG by GC
Our Services
The experienced and highly committed GBA Pharma Labs team offers a variety of different services in the following Areas.


