GBA Group Pharma Labs
Reliable support for your pharmaceutical analysis needs.
GMP Testing
Ensuring compliance with EU and FDA standards.
Decades of experience in pharmaceutical analysis.
Global customer base built on trust.
GMP Quality Control
Method Development & Validation

About GBA Pharma GmbH
The GBA Pharma Labs runs two GMP-certified and FDA-inspected sites in Germany with more than 170 highly qualified employees and over 7500 m² of lab space. These subsidiaries can draw upon decades of experience in pharmaceutical analysis and assisting with the pharmaceutical approval process in human and veterinary medicine. Over this time, they have built up a global customer base by providing reliable and faithful cooperation.
Our laboratories are certified according to EU-GMP, and US(FDA)-GMP, and we have a manufacturing license based
on §13 (1) AMG (German pharmaceutical law) as well as an import license based on §72 AMG.
Email: sales@gba-pharma.com
GMP Testing
GMP Quality Control
Method Development & Validation




Dissolution
Extractables & Leachables






Particle Determination
Elemental Impurities Testing



Regulatory Services
Amino Acid Analysis




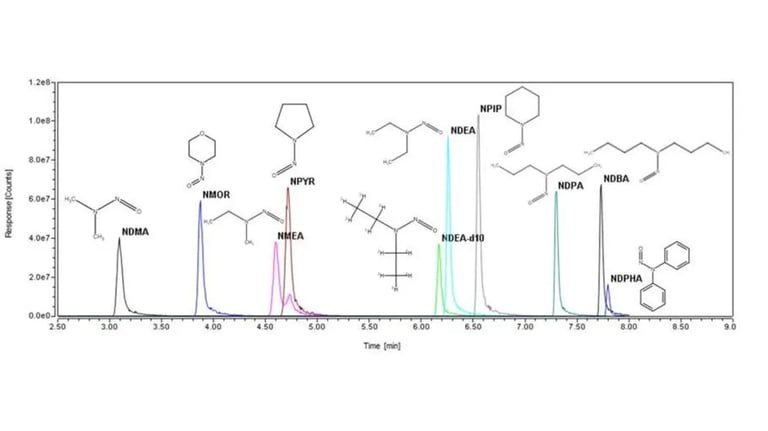
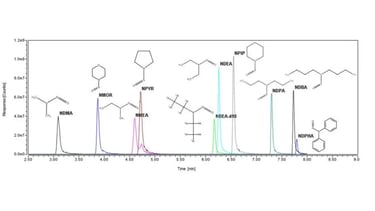
N-nitrosamines
qPCR




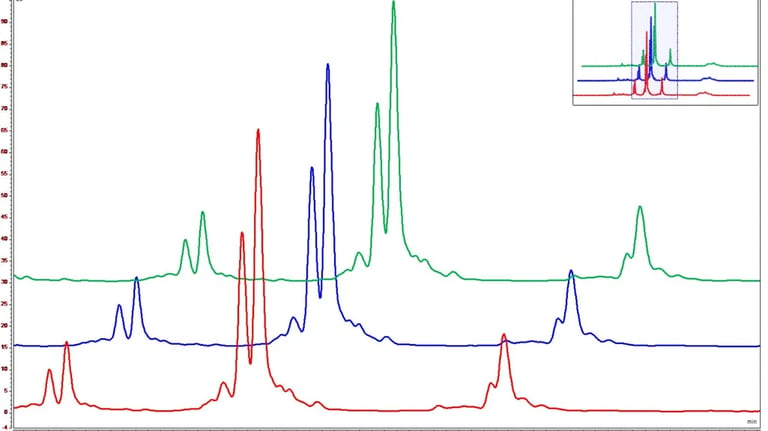
Protein Characterization


Bioassay
Stability Studies
Packaging Material Testing
Microbiology
Instrumentation
cIEF
Limit test for DEG and EG by GC
Our Services For Domestic And International Customers
Explore our services section for detailed information about services we offer

Pharmacopoeial Testing
Our Locations
GBA Group operates two GMP-certified locations in Germany, providing pharmaceutical analysis and support for approval procedures in human and veterinary medicine.
Address
GBA Pharma GmbH
Anna-Sigmund-Straße 7
82061 Neuried
Hours
Mon-Fri : 08:00 - 17:00
GBA GBA Pharma GmbH
Ernst-Abbe-Straße 40
89079 Ulm


