Antibiotic Potency Testing
Ph. Eur. 2.7.2 │ USP ⟨81⟩ – Microbiological Assays for Reliable Antibiotic Quality and Efficacy
Why Antibiotic Potency Testing is Essential
The quality, potency, and efficacy of antibiotics are critical to ensuring therapeutic effectiveness and patient safety. Regulatory authorities require precise determination of biological activity to confirm that antibiotic products meet specified concentrations and perform as intended against bacterial targets.
The microbiological assay (also known as antibiotic microbial assay) is the pharmacopoeial gold standard for measuring antibiotic potency. Compliant with Ph. Eur. 2.7.2 and USP ⟨81⟩, this method quantifies the inhibitory effect of your antibiotic sample compared to a certified reference standard.
At GBA Group Pharma, our assays support batch release, stability studies, and regulatory submissions for a wide range of antibiotics, integrating seamlessly with our comprehensive microbiology services.
How the Microbiological Assay Works
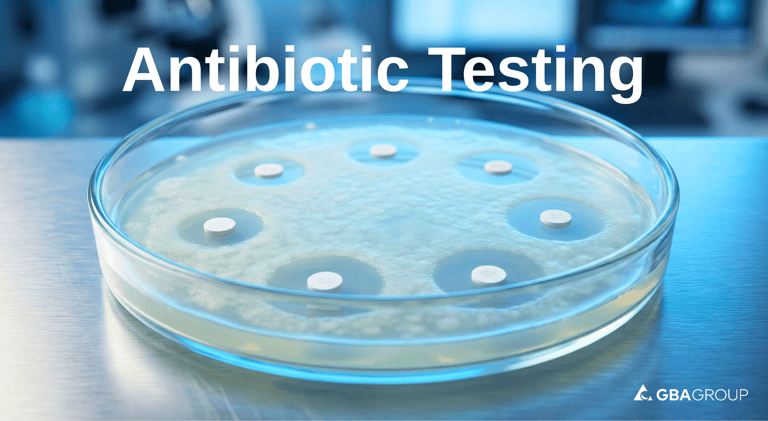
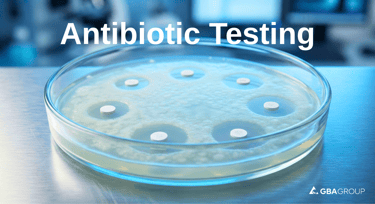
The assay relies on the agar diffusion method (cylinder-plate or disk-diffusion principles), where the antibiotic's ability to inhibit microbial growth is measured quantitatively:
Sensitive test microorganisms are inoculated into agar plates.
Sample and standard solutions are applied (e.g., via cylinders or disks).
Inhibition zones (clear areas around the antibiotic) form as the drug diffuses and inhibits growth.
Zone diameters are precisely measured and statistically evaluated against a dose-response curve for accurate potency calculation.
This bioassay ensures reproducible, biologically relevant results that chemical methods cannot replicate for antibiotics.
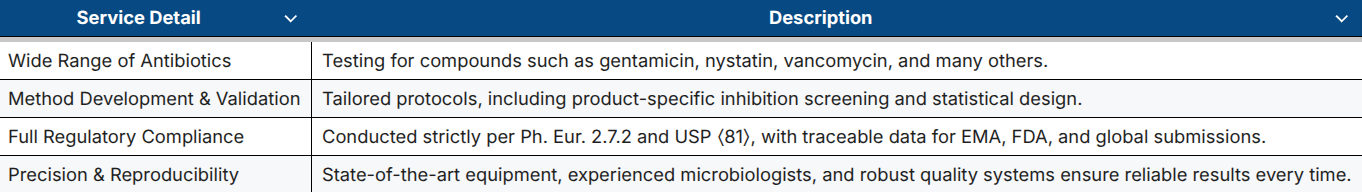
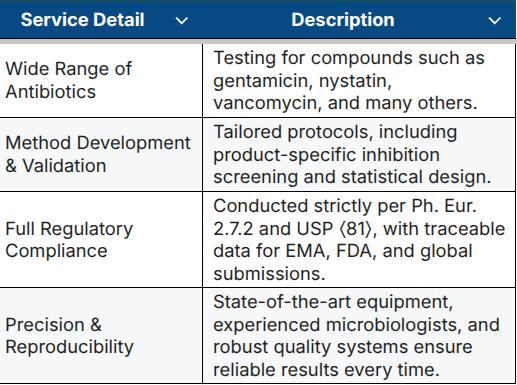
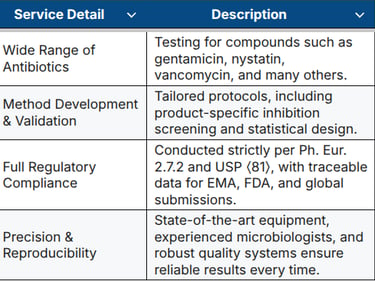
Our Antibiotic Testing Services at a Glance
GBA Group offers fully validated microbiological assays in our EU GMP-certified laboratory
Why Choose GBA Group for Antibiotic Potency Assays?
Established & Ready: Methods are fully implemented – start testing immediately without delays.
Integrated Microbiology Support: Combine with microbial limits, endotoxins (LAL/rFC), or antimicrobial preservation testing.
Expert Guidance: PhD-level support for complex assays, including troubleshooting interference or low-potency issues.
Fast Turnaround: Routine results in 3-7 days, with options for expedited release testing.
Proven Track Record: Trusted by pharma clients for accurate potency data in CTD dossiers and stability programs.
Whether for generic antibiotics, novel formulations, or biosimilars, our microbiological assays deliver the confidence you need for market approval and patient safety.
Ready to Ensure Your Antibiotic Potency?
Contact our microbiology team for a tailored quote or to learn more about transitioning to rFC in 2026.
Secure Your Quote for Antibiotic Potency Testing :
Email: sales@gba-pharma.com
Phone: +49 173 889 42 51
GMP Testing
GMP Quality Control
Method Development & Validation






Dissolution
Extractables & Leachables






Particle Determination
Elemental Impurities Testing



Regulatory Services
Amino Acid Analysis




N-nitrosamines
qPCR




Protein Characterization


Bioassay
Stability Studies
Packaging Material Testing
Microbiology
Instrumentation
cIEF
Limit test for DEG and EG by GC
Our Services
The experienced and highly committed GBA Pharma Labs team offers a variety of different services in the following Areas.


