Bacterial Endotoxins Testing
Why Bacterial Endotoxins Testing is Critical for Patient Safety.
Pyrogens are substances that can trigger inflammation or fever responses in patients, particularly when administered parenterally. Among these, bacterial endotoxins – lipopolysaccharides (LPS) from the outer membrane of Gram-negative bacteria – represent a significant risk. Endotoxins are heat-stable and can survive sterilization processes, remaining in pharmaceutical preparations even after microorganisms are destroyed.
Undetected endotoxins pose serious health threats, including septic shock in vulnerable patients. Regulatory authorities (EMA, FDA, PMDA) mandate endotoxin testing for sterile drugs, injectables, medical devices, and water for injection in accordance with pharmacopoeial standards.
At GBA Group Pharma, our bacterial endotoxins testing ensures compliance and product safety within our broader microbiology portfolio, integrating seamlessly with sterility testing, microbial enumeration, and stability studies.
Established LAL Testing Methods (Limulus Amebocyte Lysate)
The Limulus Amebocyte Lysate (LAL) test remains the gold standard for endotoxin detection, recognized across all major pharmacopoeias. Derived from the blood cells of the horseshoe crab (Limulus polyphemus), LAL reagent reacts sensitively with endotoxins to form a measurable response.
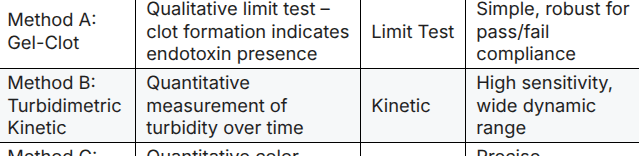
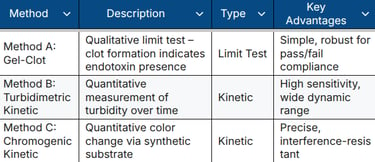
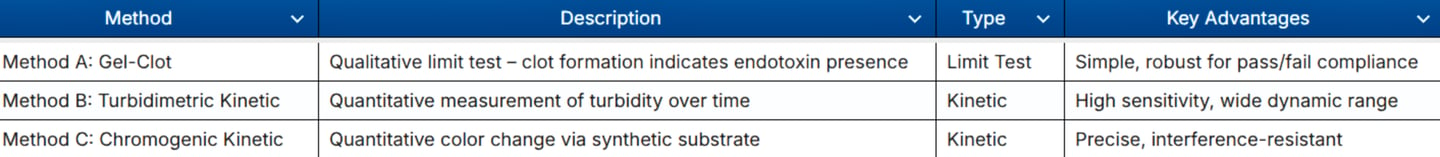
GBA Group offers fully validated LAL tests in our EU GMP-certified laboratory, compliant with Ph. Eur. 2.6.14 and USP ⟨85⟩:
Coming Soon: Recombinant Factor C (rFC) Test – A Sustainable Alternative
As part of our commitment to innovation and environmental responsibility, GBA Group will introduce the recombinant Factor C (rFC) test in early 2026.
Described in Ph. Eur. 2.6.32
Uses recombinantly produced Factor C protein (no horseshoe crab harvesting)
Animal-free, resource-efficient, and ethically superior
Equivalent sensitivity and specificity to traditional LAL
Reduces ecological impact while maintaining full regulatory acceptance
The rFC assay offers a modern, sustainable option for clients prioritizing green analytics without compromising on data integrity or compliance.
Why Choose GBA Group for Endotoxins Testing?
Immediate Availability: All current LAL methods are fully implemented and validated – no delays for your projects.
Regulatory Expertise: Harmonized with Ph. Eur., USP, and global guidelines for seamless EMA/FDA/PMDA submissions.
Integrated GMP Services: Combine with our microbiology suite (e.g., microbial limits, sterility, antimicrobial efficacy) and stability storage.
Fast Turnaround & Support: Product-specific method validation, inhibition/enhancement screening, and expert consultation included.
Future-Proof Portfolio: rFC addition in 2026 positions us as a leader in sustainable endotoxin testing.
Whether for routine QC or complex biologics, our endotoxin testing delivers reliable, traceable results to safeguard patient health.
Ready to Discuss Your Endotoxin Testing Needs?
Contact our microbiology team for a tailored quote or to learn more about transitioning to rFC in 2026.
Secure Your Quote for GMP Pharmacopoeial Testing :
Email: sales@gba-pharma.com
Phone: +49 173 889 42 51
GMP Testing
GMP Quality Control
Method Development & Validation






Dissolution
Extractables & Leachables






Particle Determination
Elemental Impurities Testing



Regulatory Services
Amino Acid Analysis




N-nitrosamines
qPCR




Protein Characterization


Bioassay
Stability Studies
Packaging Material Testing
Microbiology
Instrumentation
cIEF
Limit test for DEG and EG by GC
Our Services
The experienced and highly committed GBA Pharma Labs team offers a variety of different services in the following Areas.


