Compendial Basic Parameters Testing


Streamline your regulatory compliance with fully implemented Compendial Basic Parameters testing in our state-of-the-art EU GMP laboratory.
GBA Group Pharma delivers precise Compendial Basic Parameters testing as part of our comprehensive GMP analytical services. These routine physicochemical determinations – including density, refractive index, and osmolality – are performed under strict EU GMP conditions, with full 21 CFR Part 11 and EU Annex 11 compliance for electronic records and signatures. All methods are pre-validated, harmonized across Ph. Eur., USP/NF, and JP, and ready for immediate deployment in your batch release, stability studies, R&D, or CTD Module 3 submissions.
With decades of experience supporting global pharma clients, our tests align with ICH Q6A guidelines for specifications and EMA/FDA/PMDA requirements. Ideal for APIs, excipients, and dosage forms, these services integrate seamlessly with GBA's full GMP testing portfolio, from microbiology to dissolution. Request a quote today to accelerate your quality control without validation delays.
The Value of Compendial Basic Parameters Testing in GMP Environments
Compendial Basic Parameters testing forms the foundation of product characterization, ensuring identity, purity, and performance per pharmacopoeial standards. In 2025, with heightened focus on supply chain resilience (per EMA's GMP Annex 1 updates), these tests enable:
Rapid verification for high-volume batch release.
Cost-effective stability monitoring under ICH Q1A.
Global dossier support, including JP methods from an EU GMP site to simplify PMDA filings.
Traceable data for audits, reducing compliance risks.
At GBA, we prioritize efficiency: Most tests offer 3-5 day turnarounds, with expert interpretation to inform formulation decisions.
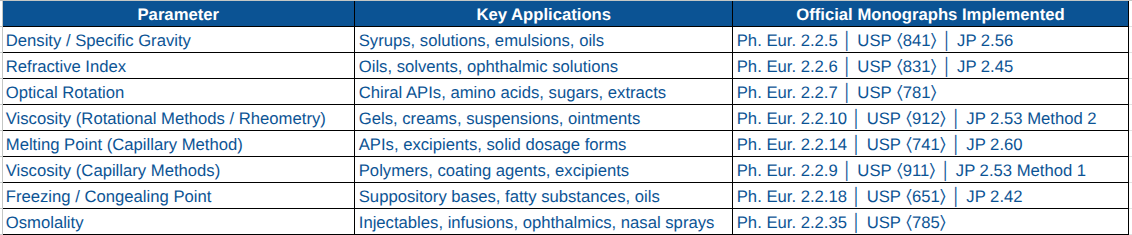
GBA's Compendial Basic Parameters Testing Portfolio (Fully Implemented & Validated)
Integrated GMP Support: Beyond Compendial Basic Parameters
As part of GBA's end-to-end GMP testing ecosystem, combine these services with:
Batch Release Testing: Full pharmacopoeial panels for market authorization.
Stability Studies: ICH-compliant storage and monitoring to complement parameter data.
Advanced Analytics: Pair with HPLC, GC, or elemental impurities for holistic QC.
This one-stop approach minimizes vendor coordination, as highlighted in our GMP services framework.
Proven Expertise: E-E-A-T in Action
GBA Group Pharma's GMP-accredited labs (ISO 17025 certified) are audited by EMA and FDA, with a track record of supporting 500+ annual projects. Our analytical chemists, backed by PhD-level validation specialists, ensure every Compendial Basic Parameters result meets pharmacopoeial rigor and regulatory scrutiny.
Frequently Asked Questions (FAQ)
Q: Are these Compendial Basic Parameters tests suitable for JP regulatory submissions?
A: Yes, all include JP monographs, performed in our EU GMP lab for PMDA acceptance.
Q: What compliance standards apply to your Compendial Basic Parameters testing?
A: Full GMP, 21 CFR Part 11 / EU Annex 11, with methods validated per USP <1225> and Ph. Eur. requirements.
Q: How does GBA ensure fast implementation for new clients?
A: Methods are pre-established – start testing within 1 week of sample receipt.
Launch Your Project with GBA Today
Leverage our validated Compendial Basic Parameters pharmacopoeial testing to meet deadlines and standards. Our GMP team is equipped for your scale – from single batches to ongoing programs.
Secure Your Quote for GMP Pharmacopoeial Testing :
Email: sales@gba-pharma.com
Phone: +49 173 889 42 51
GMP Testing
GMP Quality Control
Method Development & Validation






Dissolution
Extractables & Leachables






Particle Determination
Elemental Impurities Testing



Regulatory Services
Amino Acid Analysis




N-nitrosamines
qPCR




Protein Characterization


Bioassay
Stability Studies
Packaging Material Testing
Microbiology
Instrumentation
cIEF
Limit test for DEG and EG by GC
Our Services
The experienced and highly committed GBA Pharma Labs team offers a variety of different services in the following Areas.


